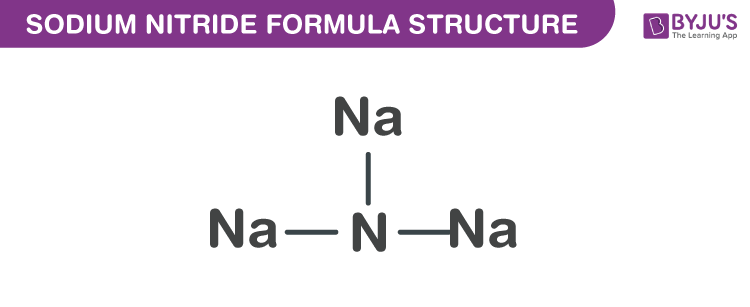
Sodium nitride formula is discussed in this article. It is an inorganic compound which is a highly unstable alkali metal nitride. It is obtained by uniting atomic beams of nitrogen and sodium deposited on a low-temperature substrate of sapphire. It has the ability to readily decompose into its elements. The molecular or chemical formula of Sodium Nitride is Na3N.
At room temperature, it is almost 90% ionic and the bandgap is that of a typical for a semiconductor. It has an anti-ReO structure consisting of the simple lattice which is made up of NNa octahedra. The N-Na bond length is 236.6 pm. The structural conformation is recorded through X-ray diffraction. The colour of the compound appears reddish-brown to dark blue and it depends on its synthesis process. It does not have a melting point.
Sodium Nitride Formula Structure
Properties Of Sodium Nitride Formula
| Chemical formula | Na3N |
| Sodium Nitride Structure | anti-ReO |
| N-Na bond length | 236.6 pm |
| Appears as | reddish-brown to dark blue colour |
| Enthalpy of formation | +64 kJ/mol |
To learn more about Sodium Nitride formula from the expert faculties at BYJU’S, register now! Also, download chemistry notes for other topics for free.
Comments